user14
1
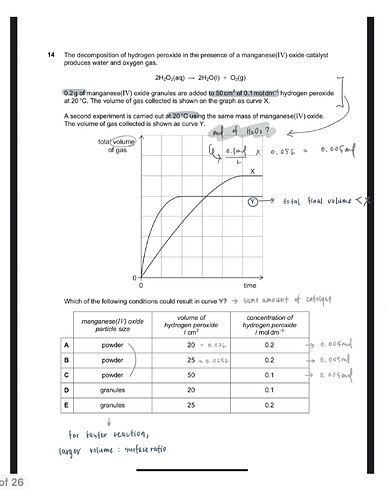
The decomposition of hydrogen peroxide in the presence of a manganese (IV) oxide catalyst produces water and oxygen gas.
2H2O2(aq) → 2H2O(l) + O2(g)
0.2 g of manganese (IV) oxide granules are added to 50 cm3 of 0.1 mol dm-3 hydrogen peroxide at 20 °C.
A second experiment is carried out at 20 °C using the same mass of manganese (IV) oxide.
The ratio of the total volume of gas is 5:4.
How can I find the volume and concentration of hydrogen peroxide of the second experiment?
*The answer is 20 cm^3 and 0.2mol/dm^3
Hi. Before I say anything, this is just how I approached to this question, and I’m not fully sure. 
We need the graph to figure out the answer since it doesn’t seem like a stoichiometry question.
The steps I took were
- Find the mol for Hydrogen peroxide, using the information presented on the question (0.005mol)
- Particle size should be powder to result in a faster
- Use the formula PV = nRT.
- We do not know any pressure, R is always constant, and T remains the same in this reaction (20 degrees). I just erased these off the equation.
- Therefore, Volume is proportional to the number of moles of hydrogen peroxide.
- According to the graph, Y has less volume than X. Therefore, Y should have less moles of H2O2.
- Then I calculated the number of moles in A, B, and C, and looked for the case which results in less moles of hydrogen peroxide.
Hope it’s correct! 
user14
3
I think you’re right!
Thank you so much