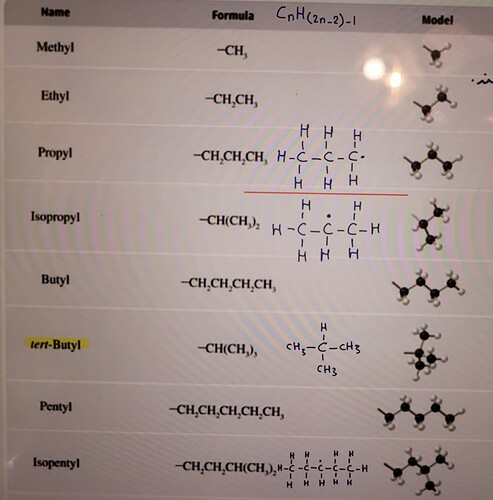
Hey!
Due to the picture we can realize that propyl is a functional group that It’s terminal carbon atom doesn’t have an hydrogen atom so as to be able to bind to the other atoms; and also isopropyl is a functional group that the middle carbon atom doesn’t have one hydrogen atom. So I thought that tert butyl is a group which has an empty hand for the connection. But in it’s formula the carbon atom has reached the octet electron configuration…why?
These are the structural isomers of propyl and we define them according to the type of alpha carbon that they have like
Propyl=primary carbon=having two hydrogen atoms directly attached
Sec propyl or iso propyl=sec carbon having one hydrogen atom directly attched to the alpha carbon
Ter butyl=ter carbon having no hydrogen atom directly attached to alpha carbon
Alpha carbon is a carbon which is directly attach to any other functional group
@Walina thank you Walina!
But in it’s formlua it’s written : CH(Ch3)3
If we want to write it’s lewis structure the alpha carbon is binded to 3 methyl groups and one hydrogen atom. So the formula which is written is wrong.
Owing to your description it should have been -C(CH3)3.
Right?
Yes the formula is wrong. It should be C(Ch3) 3. And this formula is not right because valency of carbon is 4.So there should be three methyl groups and one R group or any other new group.