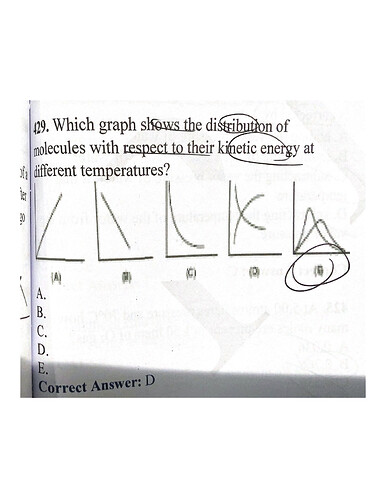
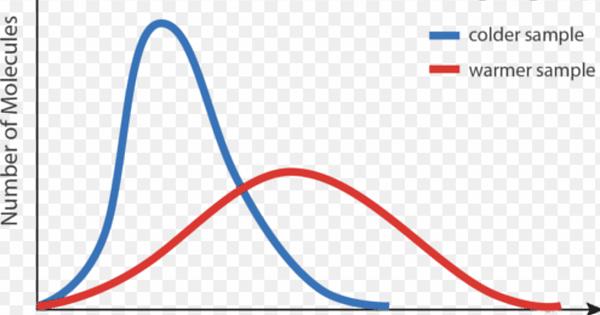
hey, the way my reasoning would be is that regardless of being exothermic or endothermic, if the solution is like this A+B ----C +D ,by increasing the temperature some molecules in right side of a reaction will turn into the left side or vice versa
the more reactants react and the number decrease , the products number of molecules increase
the kinetic energy is the temperature change in this hypothesis
please correct me if I’m wrong
@Yasy from the picture you can see that as the energy
if we consider it an exothermic and endothermic kind of graph then I think we would also add the activation energies which are not stated on D.
and this photo includes both ea and the reactions so I still don’t know why the answer key says otherwise.
oh my bad, the graph in D is indeed related to the equilibrium constant and acidic strength
sorry for the confusion