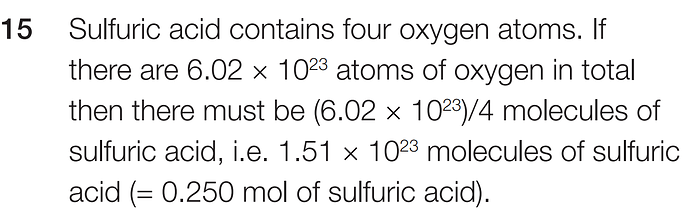hi
As the answer says, if the sample has 6,02 * 10²³ oxygen atoms, then there are (6,02 * 10²³)/4 = 1,5 * 10²³ molecules of sulfuric acid since 4 O are needed per molecule formed.
If 1 mol = Na = 6,02 * 10²³, then we have 1,5 * 10²³ * 1 / 6,02 * 10² = 0,25 moles of H2SO4
hope this helps!