how to solve this question? is it by taking antilog of -0.784 or something… Also is this level of calculation or even finding log of some number required for imat?
hey,
100/18 is not 0.18 tho? shouldnt it be 1000/18…?
You’re right my bad! i’ll let someone else answer this one
its totally fine we all make mistakes XD
Hi!
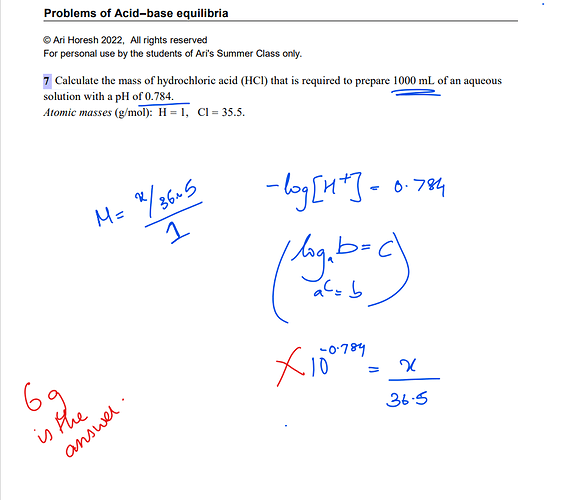
we know that pH= -log[H+].
[H+]=log^-pH=10^-0.784=0.16.
Then i used the relation:
M(concentration)=moles solute/volume solution(L)
0.16=n/1
n=0.16.
Then using n=m/Molar mass
m=n x M.M=0.16 X 36.5=6g(approximately).
I had to use calculator to find the value of [H+] also the answer was not precise i just took approximation. Hope IMAT will not ask questions involving calculator.
Hope it helps:)
hey
just to point out another way
if we have most important logarithmic equations memorized, we can conclude that log 6 =0.778========) -log 6^-1 =0.778
then the concentration would be 1/6
one mole is 36.5
36.5/6 approximately 6
thank you!
also( if at all) some log values needs to be memorized its just log 1to10 right
yes
log 1 to 7 would also suffice