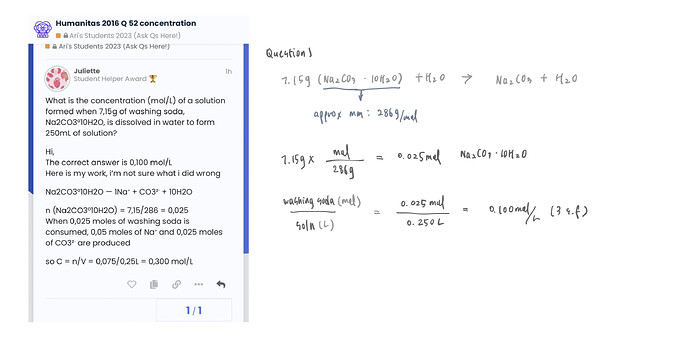
What is the concentration (mol/L) of a solution formed when 7,15g of washing soda, Na2CO3°10H2O, is dissolved in water to form 250mL of solution?
Hi,
The correct answer is 0,100 mol/L
Here is my work, i’m not sure what i did wrong
Na2CO3°10H2O — 1Na⁺ + CO3²⁻ + 10H2O
n (Na2CO3°10H2O) = 7,15/286 = 0,025
When 0,025 moles of washing soda is consumed, 0,05 moles of Na⁺ and 0,025 moles of CO3²⁻ are produced
so C = n/V = 0,075/0,25L = 0,300 mol/L