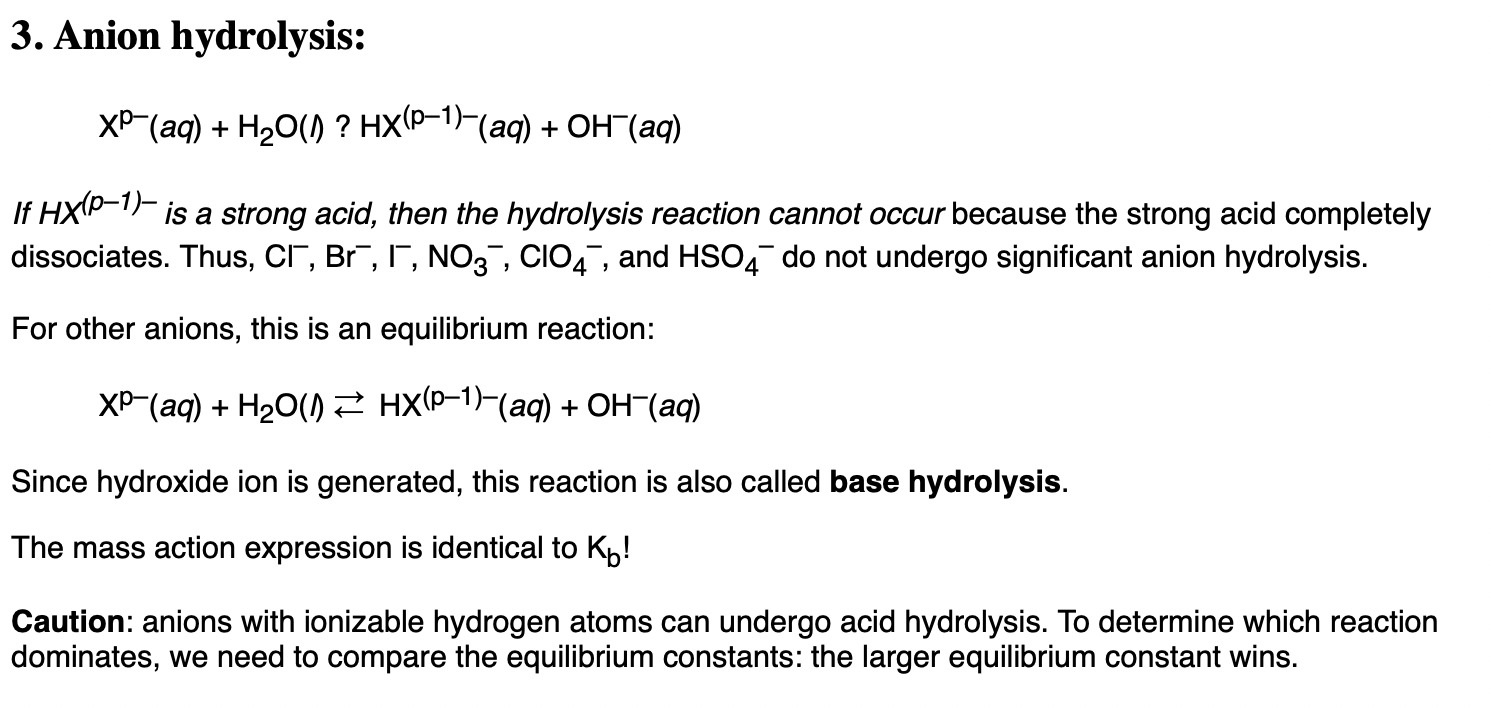
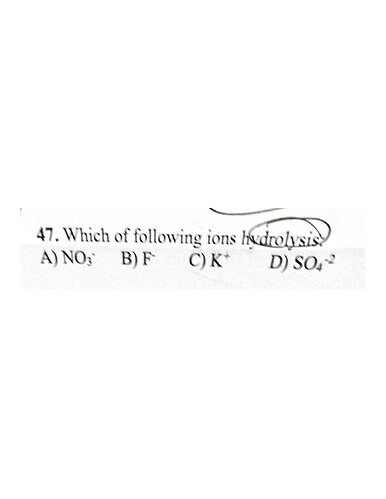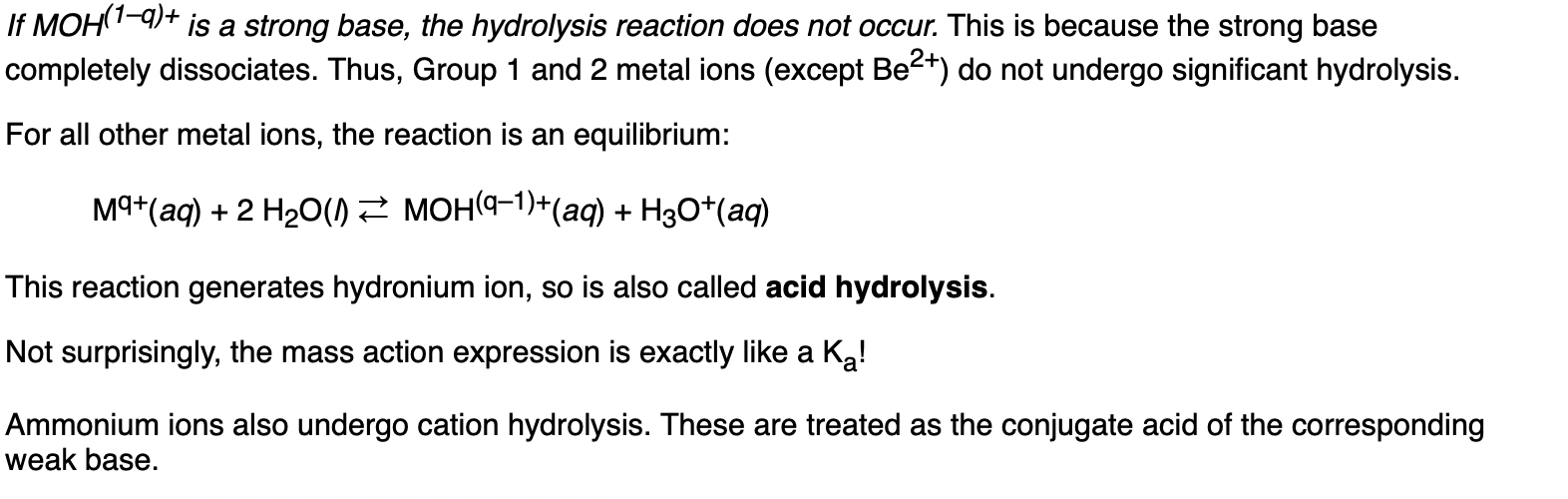Hydrolysis is the reaction with water to form an acidic/basic solution. So they’re asking which ion can hydrolyse in water. Metal ions can behave as Lewis acids. Does this explanation help with the answer?
yess thank you!! then i can derive the answer from here
What’s the answer? Is it C?
the book has no answer key so i don’t know for sure unfortunately but I was thinking A as it would be basic
According to this, I believe it’s B as its conjugate acid HF is a weak acid. Strong acids and strong bases cannot go under hydrolysis as they completely dissociate.
Hope this helps!
oh! i had no idea about this thanks for sharing, now I’m wishing that they ask this in the exam loll
so i can agree with the answer being F- for this, it would be the most sensible one.