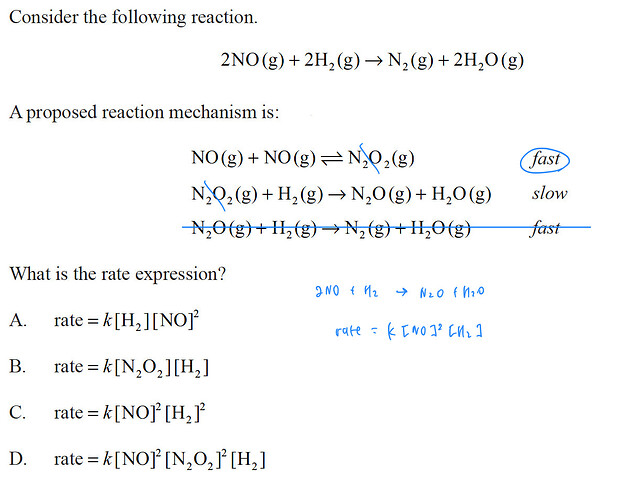answer is D
my question is, if both C and D are in the same temperature and produce same amount of moles, how do we come to the conclusion that D is the answer?
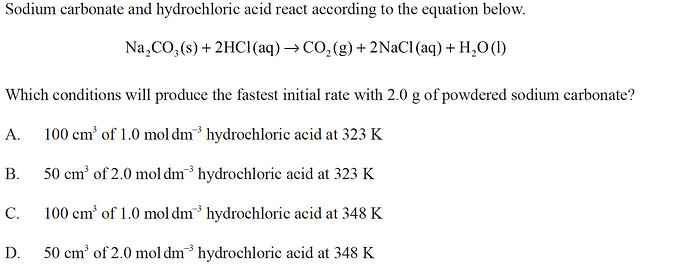
answer: A
I keep finding C as the answer…
- Rate is faster in higher molar concentration (mol/L). C < D, therefore D.
- I’m not sure about this, but I picked two reactions where we can apply Hess’s law, can someone provide more explanation?
hi
Question 1)
both options C) and D) have the same number of mols, but option C) has a lower concentration with a bigger volume. This solution is much more diluted so it will take longer for the Na2CO3 and HCl to make contact with each other and react, so option D) will produce a faster initial rate
Question 2)
the compounds N2O2 and N2O are intermediaries so we don’t have to right them in the overall equation: 2NO + 2H2 = N2 + 2H2O
rate = k [NO]^n * [H2]^m with n and m = reaction order
EDIT the rate determining step is the slowest, with the smallest reaction order
so rate = k [NO]^n * [H2]^m with n>m which eliminates option C)
N2O2 does not appear in the overall reaction, so it will not be present in the rate equation
this eliminates option B) and D)
Therefore the option must be A)
i usually use the coefficients as the rate which works but for this question it doesn’t work. there is something Im missing for sure but couldn’t figure it out
I did the same thing but why would we not care about the 2nd fast reaction?
i know that the slow rxn is the rate determining one but this has confused me.
most probably as the slow one is the second, the rest does not change the rate