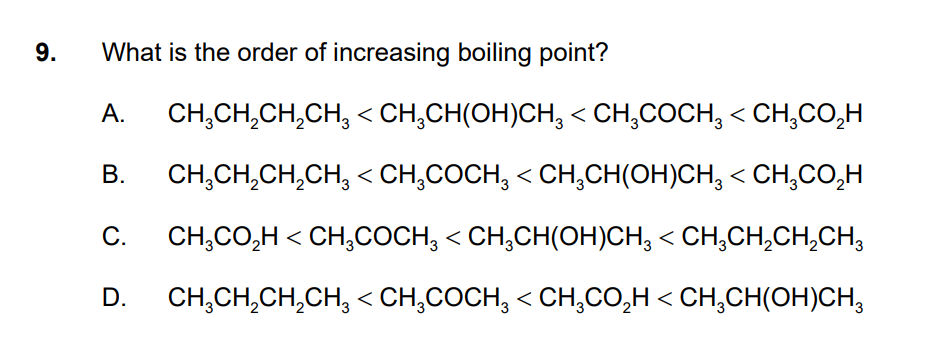
Hi
the answer is B)
i really hesitated with D)
i agree the lowest BP is CH3CH2CH2CH3 because it is non polar
the second lowest BP is CH3COCH3 becasue it is polar
CH3CH(OH)CH3 is polar with H bonds and a molecular weight of 60
CH3CO2H is polar with H bonds and a molecular weight of 60
so how do we decide which has the higher boiling point??