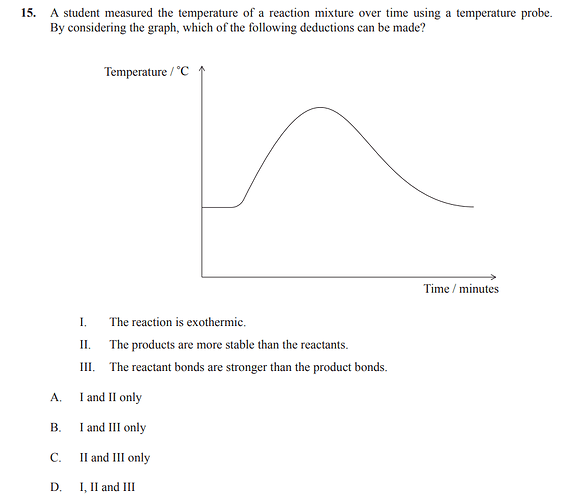
hi,
the answer is A)
i agree with statement 1 being correct
how can we see check 2 and 3 from the graph?
1 Like
Hi @Juliette
I guess since the reaction is exothermic and yet the reactant and product mixtures both have the same temperature, II is more compatible as a deduction than III. Like products can be more stable than reactants in exothermic reactions and if the reactants bonds are stronger, the graph wouldn’t show the same temperature for both reactant and product mixture.