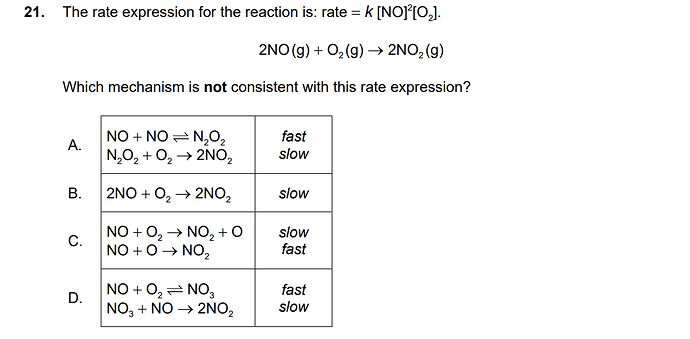hi
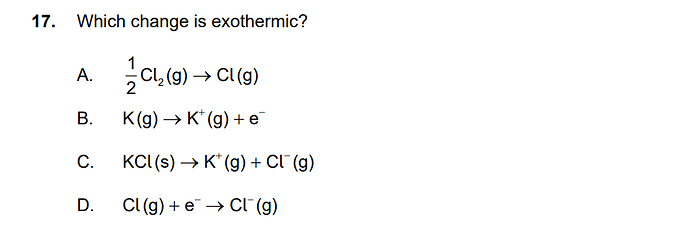
the answer for 17) is D
the answer for 21) is C
how can we solve these questions?
hello! i believe i know why but would you mind telling me which year’s questions these are so i can double check? thanks
IB chemistry november 2018
Hey, do we have to learn about the order of reactions? Is it a part of IMAT syllabus?
it is not given directly that you should learn it but solutions and equilibria is a huge part of the imat. better to know only surface knowledge is enough.
just learn how to add the solutions, how to write the rate, what is a slow mechanism.
don’t worry about it a lot, i know this topic from not the imat sources just high school
I didn’t knew about this… Thank you ![]()
@Juliette @nihantokar
Hi
I guess this would be the correct solution to 21
Rate expression is rate constant times reactants in slow reaction or rate determining step, however, in A, B & D we have slow reaction after the fast one so the reaction intermediate is in the slow reaction so we have to remove it by adding two reaction which lead to have the same rate expression as the question.
But in C, slow step is the first one, so when we write the rate expression for C u can just use the reactants in slow reaction(no addition of reactions would be unnecessary), which lead to have inconsistent rate expression as the question
okay!! thank you so much i was pretty confused with how i should use the slow reaction. i am going to edit my solution so it doesn’t confuse people