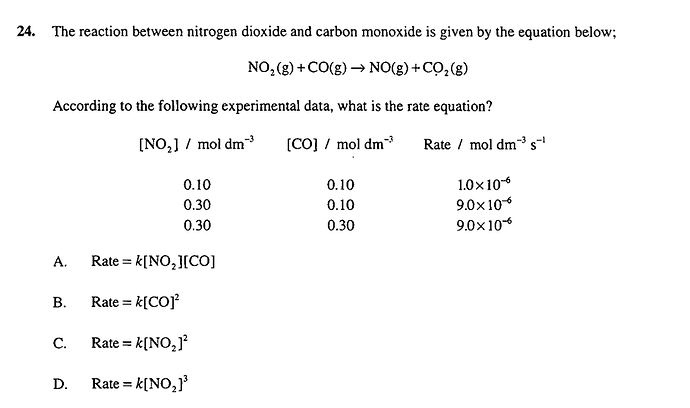
Hi, can anyone help me with this question? ![]()
Thank you very much!!!
Hi!
rate=k * [A]^m * [B]^n
to find n we look at the trials that keeps A constant
r2/r3 = (k * [A2]^m * [B2]^n) / (k * [A3]^m * [B3]^n)
1/1 = (k * 0,3^M * 0,1^n) */ (k * 0,3^m * 0,3^n)
1 = (1/3)^n
0 = n
to find m we look at the trials that keep B constrant
r1/r2 = (k * [A1]^m * [B1]^n) / (k * [A2]^m * [B2]^n)
1/9 = (1/3)^m
-2 = m (not sure why i don’t find +2???)
So rate = k [NO2]²[CO]⁰ = k[NO2]²
Is C) the correct answer?
Yup it’s C, I got it, thank you so muchhh!!! ![]()
Have a great day!!!
Yup it’s C, I got it, thank you so muchhh!!! ![]()
Have a great day!!!
Hi Juliette! I’m doing IB past papers chemistry and have some questions, if you don’t mind, can I direct message to you to ask it?
Thank you so much!!!
Yes of course! Although i don’t know how to solve a lot of IB questions i’d love to try