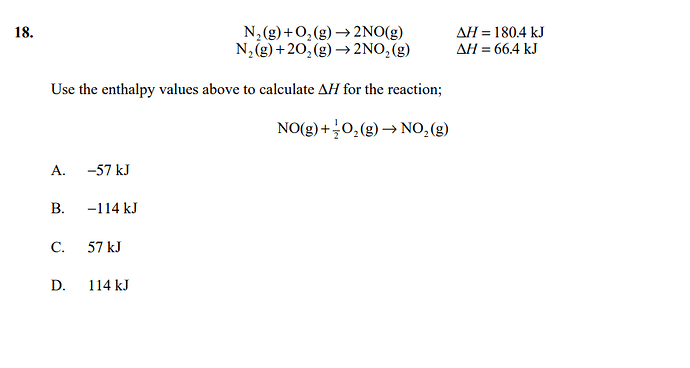Hi, can I ask why is A not B? ;;-;; I calculated B but the answer is A.
Thank you very much!!!
Hi!
Maybe since the values given are to calculate the enthalpy of reaction for 2NO+O2=2NO2 and not for NO+ 1/2 O2=NO2 so we must divide the enthalpy of products and reactants by 2.
enthalpy reaction = 1/2 enthalpy products - 1/2 enthalpy reactants = -57 kJ
2 Likes
Yup I also think like you, thank you so much!!!
3 Likes
Bro U didn’t get the stoichiometric relations correctly you just calculated energy for 2 mol instead of 1 mol so if you divide 114÷2 you get 57 as your answer