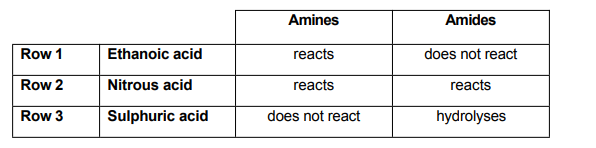
Which rows of the table correctly describe the reactions of the aqueous acids with amines and amides?
A. Rows 2 and 3
B. Rows 1 and 3
C. None of the rows
D. All of the rows
E. Rows 1 and 2
Which rows of the table correctly describe the reactions of the aqueous acids with amines and amides?

A. Rows 2 and 3
B. Rows 1 and 3
C. None of the rows
D. All of the rows
E. Rows 1 and 2
Let’s first discuss what we know about each substance mentioned:
Ethanoic acid is a weak carbonic acid, while both nitrous and sulphuric acids are strong acids.
We can also say that between amines and amides, amines are more reactive- they’re actually one of the most reactive nucleophiles.
So we could say that all these acids should be able to react with Amines. Immediately we can see Row 3 is incorrect. In fact, Sulphuric is a strong acid- and in reaction with an amine it should hydrolyze.
Row 1 checks out, as the weaker amide base would not be able to react with a weak acid.
Row 2 checks out as well, since nitrous acid is a stronger acid- it should have no issues reacting with a weaker base.
Our answer is therefore, Rows 1 and 2, option E.
Hello, thanks for the explanation. Regarding nitrous acid, I’ve read that it is actually a weak acid while nitric acid is a strong acid. I’ve been confused by this question since many websites and textbooks say different things. ( like for amides, some says it is a weak acid while the other says it’s a weak base or neutral)Could you please clarify that?
Anyone be able to help on this one? Still a bit confused on the answer