Hi!
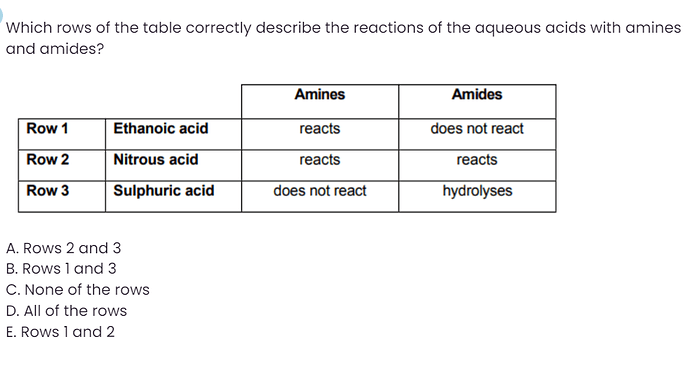
Row 1 is correct as ethanoic acid (a weak acid) will react with a strong base like an amine but not a weak base like an amide
Row 2 is correct as nitrous acid (weak acid) will react with an amine to form a salt. Despite being a weak acid, nitrous acid will react with an amide to produce carboxylic acid, nitrogen gas and water
Row 3 is incorrect, as sulphuric acid (a strong acid) will react with strong base. (it does also cause hydrolysis but the row is still incorrect).
Hope it helps!
1 Like
Thank you for your precise explanation. it was helpful indeed!
1 Like