Which rows of the table correctly describe the reactions of the aqueous acids with amines and amides?
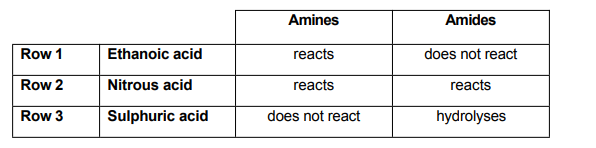
A. Rows 2 and 3
B. Rows 1 and 3
C. None of the rows
D. All of the rows
E. Rows 1 and 2
Which rows of the table correctly describe the reactions of the aqueous acids with amines and amides?

A. Rows 2 and 3
B. Rows 1 and 3
C. None of the rows
D. All of the rows
E. Rows 1 and 2
Amines => Strong bases
Amides => Weak bases
Ethanoic acid => weak acid
HNO3 & H2SO4 => Strong acids
Row 1 is correct because weak acid can react only with strong base but can not react with a weak base.
Row 2 correct because strong acid can react with both strong or weak base.
Row 3 incorrect because of the first part. Sulphuric acid is strong acid so should react with strong base.
So, correct answer is E.
hey ![]() isn’t nitrous acid a weak acid ?
isn’t nitrous acid a weak acid ?
YES nitrous acid is a weak acid it is HNO2 not nitric acid which is HNO3.
the problem with this question, i think, is the exceptions. now we know that strong acids react with both strong and weak bases typically and weak acids doesnt react with weak bases typically again.
and by the way amines are not strong bases!! the only strong bases are group 1 and 2 hydroxides.
anyways that was probably the hardest Q in chemistry so if u face such Q it is okay if u dont answer it because you really dont have to answer everything.
good luck everybody
O my bad, I misread it as nitric acid.
Yes nitrous acid is a weak acid.
I think we can guess the reactivities by the relative values of ka of Sulphuric acid, nitrous acid and ethanoic acid. I am not sure if it will work.