The positions of the main group elements in the Periodic Table are shown below:
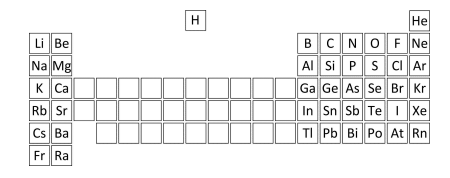
Which one of the following formulae is NOT correct?
A. GaCO_3
B. CsNO_3
C. BeSO_4
D. SnS_2
E. Ba(HCO_3)_2
The positions of the main group elements in the Periodic Table are shown below:

Which one of the following formulae is NOT correct?
A. GaCO_3
B. CsNO_3
C. BeSO_4
D. SnS_2
E. Ba(HCO_3)_2
When looking over each compound we can see:
Gallium and a carbonate ion should not be able together in a 1:1 ratio. This is because Gallium is not able to form a 2+ ion, but rather a 3+ ion. And since we know carbonate ions have a valency of 2-, our compound should instead be:
Ga_2(CO_3)_3
And therefore, our answer is A.