Hi!
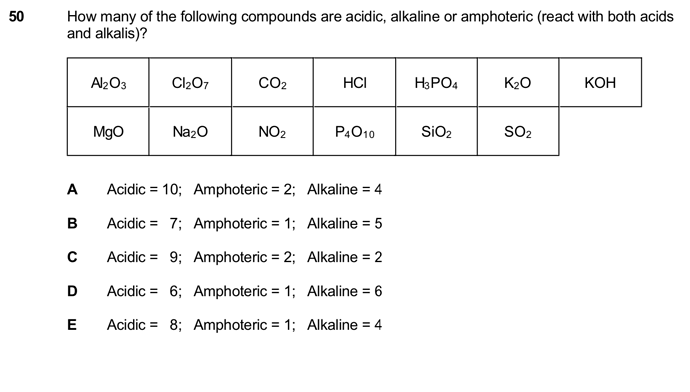
1 Amphoteric: Al2O3
4 Alkaline: KOH, K2O , MgO and Na2O
8 Acidic: Cl2O7, CO2, HCl, H3PO4, NO2, P4O10, SiO2, SO2.
Answer is thus E.
Hope it helps:)
What’s the process of solving this one?
Hi!
Having a general idea of oxides of non metals and metals would help us solving this question.
As we know that metal oxides are basic in nature (exception. Al2O3: Amphoteric) and non- metals oxides are acidic (exception: NO,CO and N2O: these are neutral oxides) I think knowing it as general rule while keeping in mind of the following exceptions will helps us tackle similar questions.
Hope it helps:)
Oh, ok thanks! Why is Al2O3 amphoteric? Is that just a rule about aluminum?
Hi!
The reason Al2O3 being amphoteric because it can react both with acid and bases ![]()