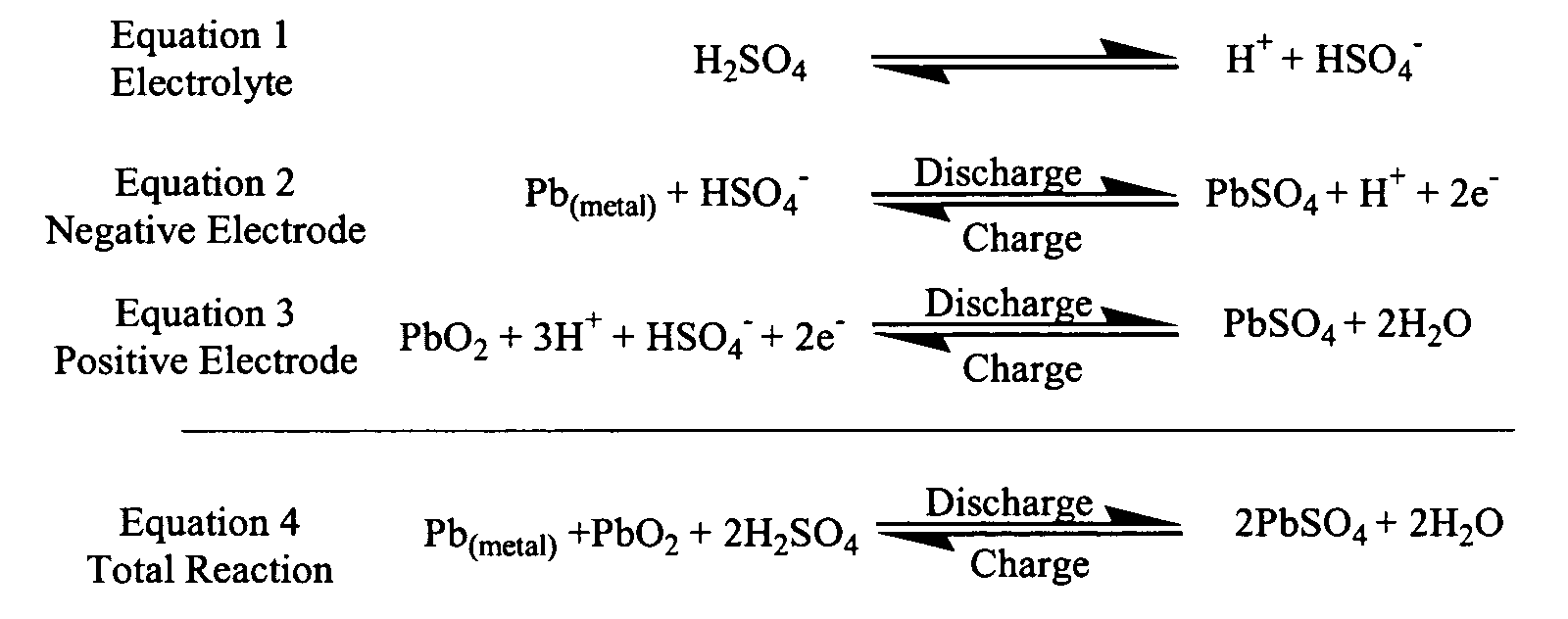
This question approaches the idea of a Lead-acid storage battery. Let’s understand this idea.
First, we must note what exactly should happen at the point of discharge and recharge. During discharge, a redox reaction takes place as a result of the electrical energy produced.
This means one substance must undergo oxidation (loss of electrons) and another must undergo reduction (gaining of electrons).
Charge:
When the sulfuric acid dissolves, its molecules break up into positive hydrogen ions (2H^+) and sulphate negative ions (SO_4^{2—}) and move freely.
The hydrogen ions, being positively charged, moves towards the negative electrode (cathode).
The SO_4^{2—} ions being negatively charged moved towards the positive electrode (anode).
Each hydrogen ion takes 1 electron from the cathode, and each sulphate ion takes the 2 negative ions from the anodes and react with water to form sulfuric and hydrogen acid.
The oxygen, produced from the above equation, reacts with Lead Oxide to form Lead Peroxide (PbO_2). Thus, during charging the Lead Cathode remains as Lead, but the Lead Anode gets converted into Lead Peroxide.
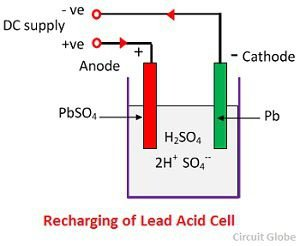
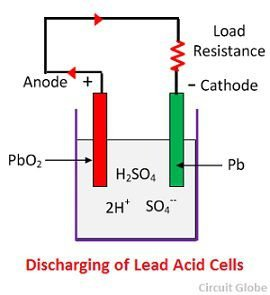
Discharge:
When the cell is in discharge, the anode is covered in Lead Peroxide (PbO_2) and the cathode is of metallic sponge lead (Pb).
When the electrodes are connected through a resistance, the cell discharge and electrons flow in a direction opposite to that during charging.
The hydrogen ions move to the anode and receive 1 electron from the anode and become hydrogen atoms.
The hydrogen atom reacts with PbO_2, and forms lead sulphate PbSO_4. Lead has gone from an oxidation number of +4 to an oxidation number of 2+/
Each sulphate ion (SO_4{2—}) moves towards the cathode gives up 2 electrons, becoming SO_4, which reacts with the metallic lead cathode and forms Lead Sulphate.
Lead has therefore been oxidized from it’s element form (0) to it’s compound form in Lead Sulphate (2+).
Our answer is therefore, B.