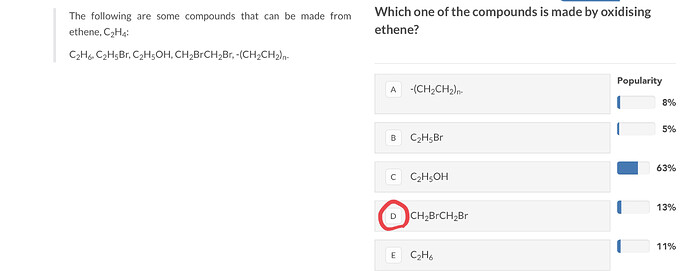
This question is basically about oxidation state.
Something is being ocidised if its oxidation stete is increasing (meaning it is losing electrons)
In the first compound (from the left) the oxidation state is being decreased so it is being reduced.In the second, the third and the fifth one the oxidation state isn’t changing. And only in the fourth compound the carbon’s oxidation state is being incresed from -2 to -1.
1 Like
Oh…
I didn’t consider the oxidation number😂
thank you so much