Hi! You can search up the question in the search bar, most IMAT past paper questions have already been solved, it should be there :))
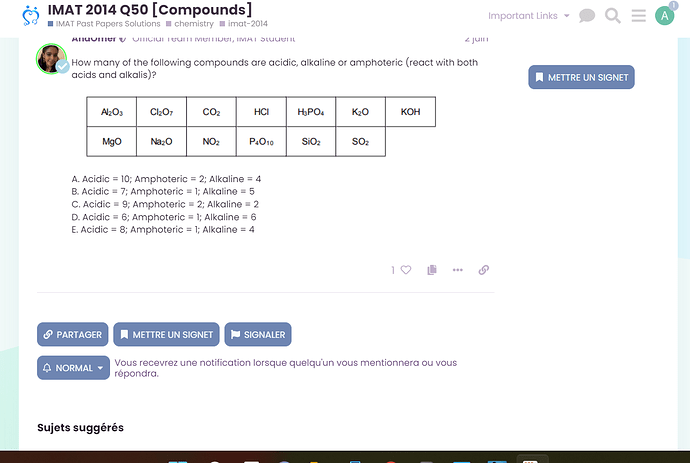
Hello again ![]() that’s what I’m usually used to do, but i can’t seem to find the solution to these questions! Eevery time I type it in the search bar I only have the question but not the soltion. Below is a sceenshot.
that’s what I’m usually used to do, but i can’t seem to find the solution to these questions! Eevery time I type it in the search bar I only have the question but not the soltion. Below is a sceenshot.
Oxidation is LOSS of electrons, so when we make a saturated compound (only single bonds) from an unsaturated one (double bonds), we LOSE electrons.
Therefore, when ethene reacts with bromine gas, an electrophilic addition reaction occurs where bromine atoms attack the double bond and take the electrons in order to form a bond with carbon atoms.
It follows that the formation of dibromoethane is an oxidation of ethene, because previously there were 4 shared electrons in the double bond between carbons (C=C), but now there are only 2 (C-C), so the molecule has theoretically been oxidised.
So therefore the double bond breaks and joins to the halogen, in this case: bromine. Let me know if you need anything else
This explanation was made by @DariusDuhan but I just copied it for you here