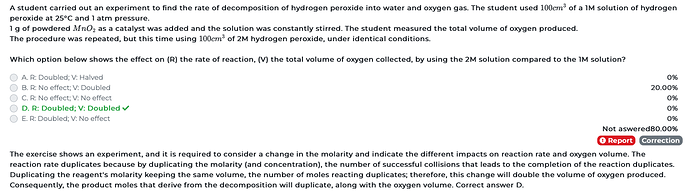
This question got me, can some please help here!? Thanks a million, you guys frickin rock
Hi! So these are the changes between the two reactions
concentration X2
Other conditions stayed the same. So we know that when the concentration doubles, the number of moles of the reactant has doubled per unit volume. Since there is twice the number of reactant molecules, more collisions occur with the catalyst per unit time and therefore the rate doubles. (proportional increase)
Also when there are more moles of the reactant there should be more moles of the product, it should change proportionally. Therefore the volume of oxygen also doubles
2H2O2 → 2H2O +O2
Is it fair to say if the concentration were to quadruple? The moles produced & rate would quadruple?
Concentration being directly proportional.
Quadruple means times by 4 so that would be incorrect since the concentration only increase by a factor of 2. The right word would be doubled!
haha yes, I was using it as a hypothetical scenario, IF the concentration where to quadruple - then the moles would quadruple as well…it is a 1:1 / 2:2/ 4:4 relationship?
Oh! Yes that’s true :))