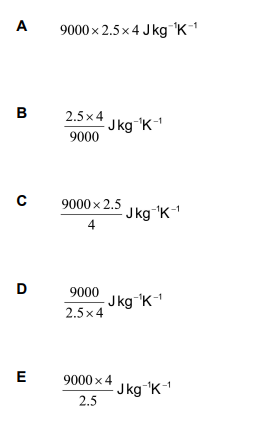
An aluminium block of mass 2.5 kg is supplied with 9000 J of thermal energy. This causes its temperature to rise by 4 K. Which expression gives the specific heat capacity of this aluminium, from this data?
[Assume that the block remains solid throughout, and that no additional energy is exchanged between the block and the surroundings.]
This question requires the simple knowledge of this formula:
E=mc\Delta t, where
E= Thermal energy supplied (J)
m= mass (kg)
c= specific heat capacity (J/kgK)
t= temperature (k)
We can now simply rearrange the formula to make (c) the subject, then plug in the given numeric to find the appropriate answer.
c= \frac{E}{m\Delta t}= \frac{9000}{(2.5)(4)} Jkg^{-1}K^{-1}
Our answer is therefore D.
4 Likes
