The dipeptide represented below is in aqueous solution.
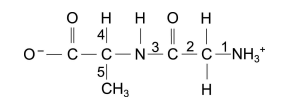
Which numbered bond (1 to 5) needs to be broken (hydrolysed) in this dipeptide to directly form two amino acids?
A. 1
B. 5
C. 3
D. 4
E. 2
The dipeptide represented below is in aqueous solution.

Which numbered bond (1 to 5) needs to be broken (hydrolysed) in this dipeptide to directly form two amino acids?
A. 1
B. 5
C. 3
D. 4
E. 2
The correct answer is C.
Amino-acids are organic compounds that are composed of 2 distinct groups : one is invariant, and the other one, called the R-group, is specific to each amino acid. The invariant group, common to all amino acids, is made of 2 functional groups :
Amino or -NH2
Carboxyl or -COOH
They are both attached to an atom of carbon, that we will call Cα ; that same atom is linked to a single atom of hydrogen and the R-group.
To answer this question, you have to be able to identify the amino and carboxyl functional groups on the molecule.
Once you get that, you can determine that you have to break the bond between N and C at the “3” position to form 2 amino acids, like the following :
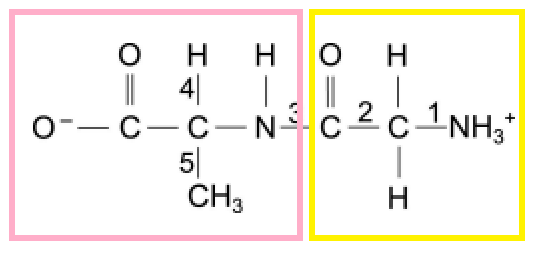
Hey, doesn’t the second structure (yellow) only have 1 oxygen, while for a caboxylic group 2 oxygens are needed?
the OH from the carboylic acid was removed to connect the two amino acids in the diagram
to break the bond we’ll have to hydrolyze the compound, so the O from H2O will take care of that missing second oxygen.
There is no H on COO of left group.
For carboxylic group you need COOH.
And even if we put water hydrogen won’t get there, it will only connected to the end which is cut. Isn’t ir?