A mixture of methanol and ethanoic acid is left until equilibrium is reached. The equation for this reaction is given below.
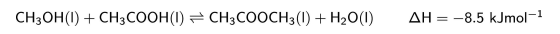
The amount of CH_3OH in this mixture at equilibrium can be increased by:
- adding more water to the mixture
- raising the temperature of the mixture
- adding sodium hydroxide to the mixture
- adding a catalyst to the mixture
A. 2 and 4 only
B. 1 and 2 only
C. 1 and 3 only
D. 1, 2 and 3 only
E. 4 only
4 Likes
This question is designed to test the knowledge concerning the equilibrium of reversible reaction.
1.adding more water, or in a nutshell increasing the concentration of products. When increasing the concentration of products, the equilibrium would shift to left to resist the effect brought by this change.
2.raising temperature., when discussing the temperature, we have to check the enthalpy given in this question. H<0–exothermic reactions.Therefore when putting more heat to the closed system, it would prefer the endothermic reaction and the equilibrium would shift to the left.
3.adding NaOH to the mixture, the NaOH would ionise into more OH- and ph would increases and the equilibrium would shift to left to produce more acid to resist the change.
4.adding a catalyst. Tricky ones,the catalysts would increase the forward and backward reaction speed and the equilibrium would not shift and stay constant.
1 Like
![]()