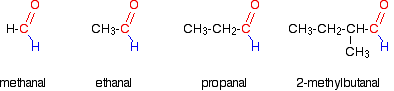Intermolecular forces are defined as the attractive or repulsive forces present between atoms, molecules, or ions of a substance when they are placed close to each other.
They control the physical properties of substances, such as melting and boiling points. The size of the boiling point is hence governed by the strengths of the intermolecular forces.
Ethanal:
Ethanal is an aldehyde, which we know are polar molecules because of the carbon-oxygen double bond.
Oxygen is far more electronegative than carbon and so has a strong tendency to pull electrons in the bond towards itself.
One of the two pairs of electrons that make up a carbon-oxygen double bond is even more easily pulled towards the oxygen. That makes the carbon-oxygen double bond very highly polar.
As well as the dispersion forces, there will also be attractions between the permanent dipoles on nearby molecules. That means that the boiling points will be higher than those of similarly sized hydrocarbons - which only have dispersion forces.
This is the case with propane.
Propane:
The only intermolecular forces in propane are London dispersion forces. Because propane is a small molecule, these dispersion forces are also small, and so not much energy is needed to break them.
At room temperature, the molecules have too much energy for these weak forces to hold them together as a liquid.
This makes propane a weaker compound, and definitely weaker than ethanal.
Carbon Dioxide:
Since O is more electronegative than C, the C-O bond is polar with the negative end pointing toward the O.
CO has two C-O bonds. The dipoles point in opposite directions, so they cancel each other out.
Thus, although CO₂ has polar bonds, it is a nonpolar molecule. Therefore, the only intermolecular forces are London dispersion forces.
Hence, only London forces or Dispersion forces are present as intermolecular forces in CO2.
In increasing the strength of intermolecular forces, which leads to increased strength in boiling points, our order should therefore be:
CO_2 , C_3H_8, CH_3CHO. Which is option A.
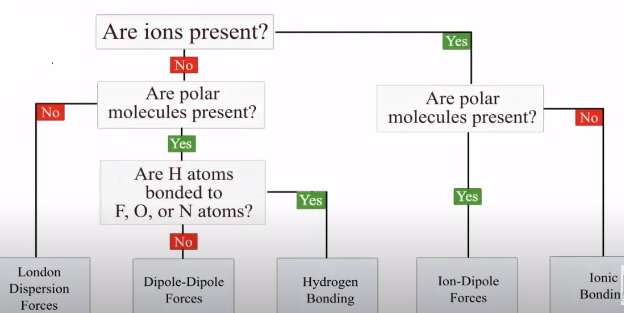
This is a good guide to utilise when solving for intermolecular forces: