Background
To determine the answer, we need to find the intermolecular forces of the molecules.
Example:
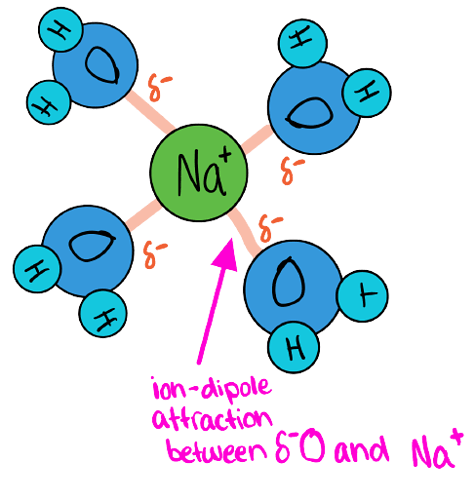
NaCl : which is an ionic salt. It is made of 2 ions Na^+ and Cl^- which will interact to form ion-dipole attractions with water.
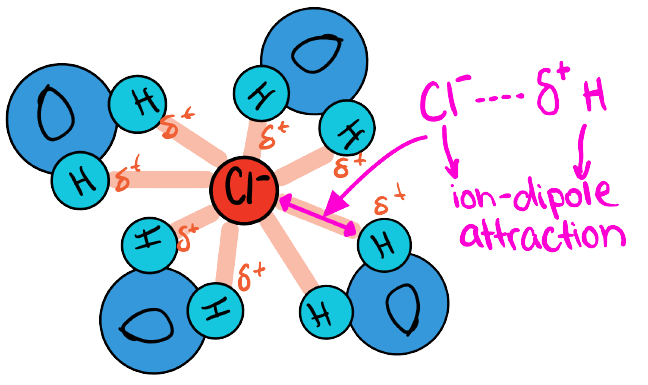
What Happens with Cl^- ion
(-) so attracted to (δ+)
What Happens with Na^+ ions
(+) attracted to (δ-) part of water (oxygen atom)
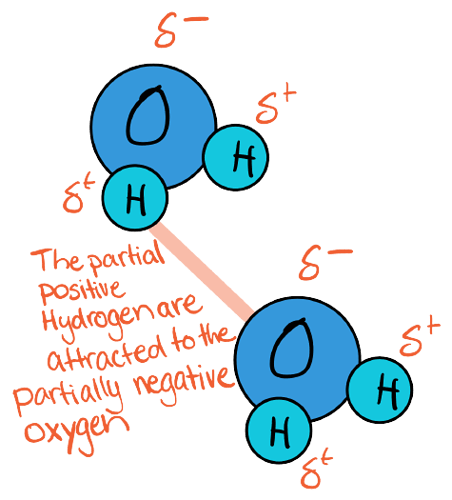
Oxygen has a higher electronegativity than Hydrogen so there is a net-dipole moment. Oxygen exerts a stronger attraction on electrons in water’s covalent bond between O-H. As a result, the electrons spend more time around oxygen and less time with Hydrogen. This separation of charge makes oxygen partially negative (δ-) and hydrogen partially positive (δ+)
Partial negativity (δ-) is caused when the electrons spend MORE time around the atom than in other regions.
Partial positivity (δ+) is caused when the electrons spend LESS time around the atom than in other regions.
Approach
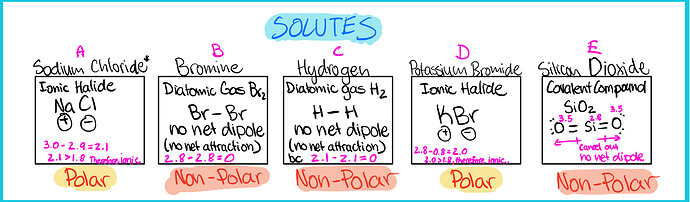
- Find the polarity of solutes
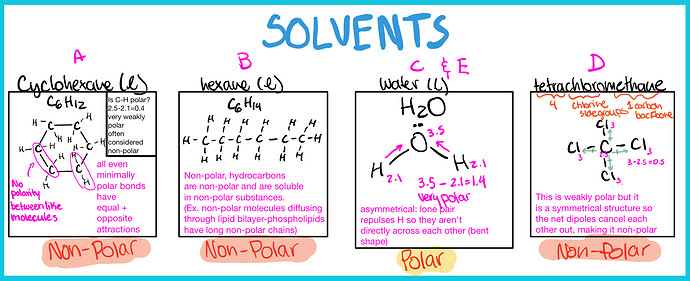
- Find the polarity of solvents
- Find the strength of intermolecular forces (not needed here - more complex)
Polarity is determined by the structure of the molecule (asymmetric = polar), and the difference in electronegativity
Electronegativity Scale
0.0-0.4 (non-polar - covalent bond)
0.4-1.7 (polar - covalent bond)
greater than (>) 1.7 (Ionic bond)
Remember that Like Dissolves like!
Let’s summarize the results:
A. Polar solute ------- Non-polar solvent
B. Non-Polar solute ------- Non-polar solvent
C. Non-Polar solute ------- polar solvent
D. Polar solute ------- Non-polar solvent
E. Non-Polar solute ------- polar solvent
\fcolorbox{red}{grey!30}{Therefore the answer is B, Bromine in liquid hexane.}