What are the 3 most basic rules in nomenclature for organic compounds (IUPAC)?
- Identify the longest straight carbon chain,
- Identify the functional group (determines suffix),
- Identify the side chains of substituent groups (prefix) (name in order ex. 1-bromo, 2-methyl… If 2 on same carbon use alphabetical naming)
Number of carbons in longest Chain - Stem in IUPAC name
Meth- 1
Eth- 2
Prop- 3
But- 4
Pent- 5
Hex- 6
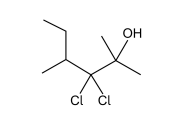
So here we have a 6 carbon-long compound with 5 different side groups. The main functional group is alcohol, and we can determine it is the principal functional group because it has the highest priority.
Lowest to Highest Priority Functional Groups
(Lowest) alkyl < alkyne < alkene < amine < alcohol < ketone < aldehyde < nitrile < amide < carboxylic acid (highest)
From the backbone, we know we will use: hex-
From the functional group, we know the suffix is: -ol
Remember that we must number the carbon backbone with the highest priority side chain having the lowest number, and numbering in the way that gives you the lowest possible number. (Ex. 2-chlorohexane instead of 5-chlorohexane)
The functional group can either be considered on carbon 5 or 2 in this case, so we will choose 2 since it is smaller.
This gives up hexan-2-ol.
Now for the side chains: We have 2 chlorine groups and 2 methyl groups. We will need prefixes for these, and since there are 2, we will call them dichloro- and dimethyl-. Since we already have a direction for naming (-OH is on the second carbon), we can follow this numbering, resulting in 3, 3-dichloro and 2, 4-dimethyl. Now we have all the pieces and just need to arrange them.
How do we determine whether chloro- or methyl- is named first? They are named in alphabetical order (excluding the prefix - di in this case).
C before M so chloro- first.
Now assemble:
3, 3-dichloro-2, 4-dimethylhexan-2-ol
\fcolorbox{red}{grey!30}{Therefore the answer is A.}
Note: this was just briefly giving the reasoning behind our choices in naming, but you should review IUPAC rules and practice on your own in order to follow and understand these steps