Have a question you need some help solving? Ask them here!
Please try to upload the questions in text format, if the question is a picture, try to transcribe it.
If you have problems uploading images, just go to https://imgur.com/, upload your pictures them embed them here!
1 Like
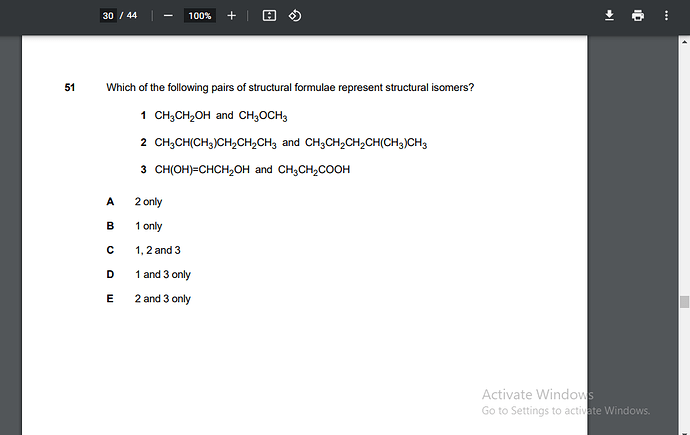
The correct option is D.
You might be confusing with 2 that one actually the same compound so it can not be structural isomer. You can check this by naming the compound by IUPAC that is 2-methly pentane.
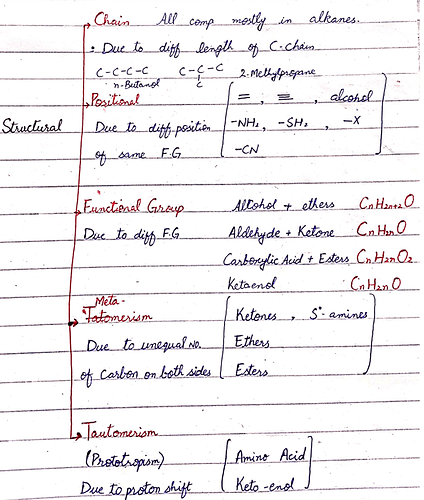
I am attaching the summary of the structural isomer that might be helpful for you.
4 Likes
Thank you so much. Could you also explain why 3 shows structural isomerism?
CH(OH)=CHCH2OH (Functional groups=> Alcohol and alkene)
Molecular formula: C3H6O2
CH3CH2COOH (Functional group=>carboxylic acid)
Molecular formular: C3H6O2
As both have same no of atoms and molecular formula and different structures so they are structural isomers.