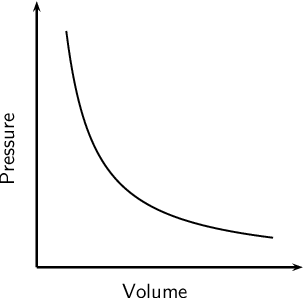
A fixed mass of an ideal gas is compressed at constant temperature. The pressure is recorded continuously as the volume decreases. The pressure ( y-axis) and volume (x-axis) are plotted on a linearly scaled graph.
Which statement describes the plotted line?
A. a curved line of increasing positive gradient starting at the origin of the graph
B. a curved line with negative gradient of decreasing magnitude
C. a straight line parallel to the pressure axis
D. a straight line parallel to the volume axis
E. a straight line of positive gradient starting at the origin of the graph