I don’t understand why 2 is not correct. Aren’t structural isomers suppose to have the same molecules but different structures. Can someone explain it to me?
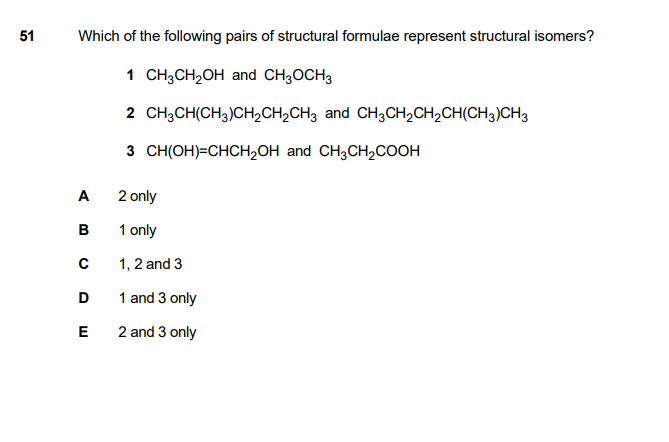
I don’t understand why 2 is not correct. Aren’t structural isomers suppose to have the same molecules but different structures. Can someone explain it to me?

Hi!
Hope this helps!