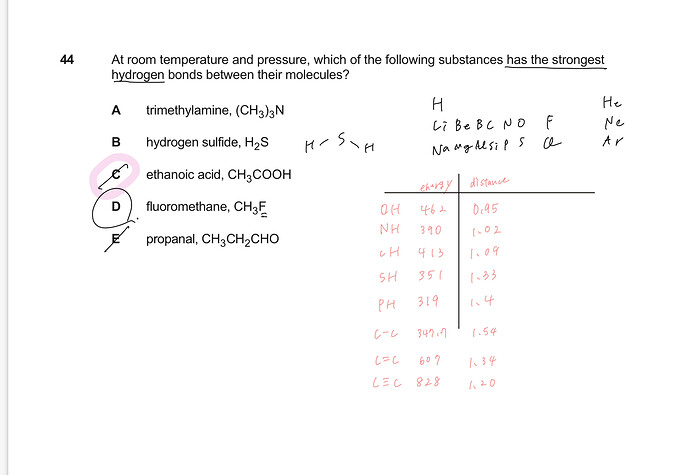
How do I solve this question? Do I need to memorize binding energy?
Hi!
Trimethylamine: no hydrogen bonding as there is no N-H bond
Hydrogen sulfide: no hydrogen bond (hydrogen bonds do not happen for H-S bonds)
Ethanoic acid: hydrogen bond present as there is an O-H bond
fluoromethane: no hydrogen bonding since the F atom is bonded to the central C atom (F must be bonded to H in order for hydrogen bonding to occur)
propanal: no hydrogen bond as there is no O-H bond (we have instead a carbon double bond to oxygen)
As such the only option is ethanoic acid.
Hope it helps:)
8 Likes