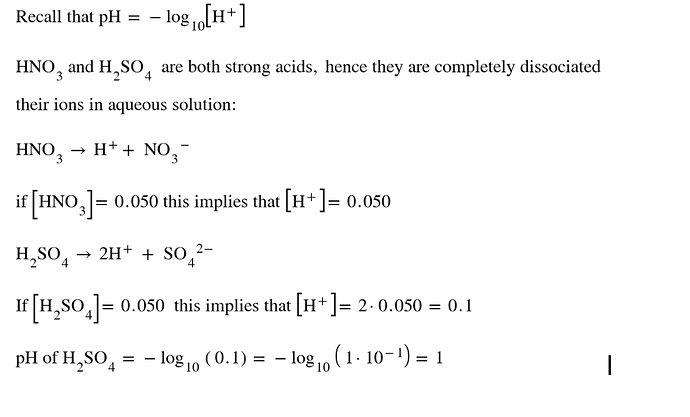In this question, it is obvious that options 1 and 2 are not valid choices. However, with respect to option 3,how can we solve -log(0.5)?
1 Like
You don’t have to calculate that. H2SO4 is a strong acid so it dissociate completely in water. for every mole of H2SO4 you will get two moles of H+ so your H+ concentration in the solution would be 0.1 Mol L^-1, which obviously gives us a pH of 1. But let’s just for the sake of your question try to calculate -log(0.5) and well… it’s impossible without a calculator. Actually the answer is 0.301. But I’ll give you a tip. Remember that 0.5 is a number between 1 and 0.1, if -log(1) gives us a 0 and -log(0.1) gives us a 1 than -log(0.5) must give us a number between 0 and 1!
6 Likes
I don’t understand how did we get 0.5