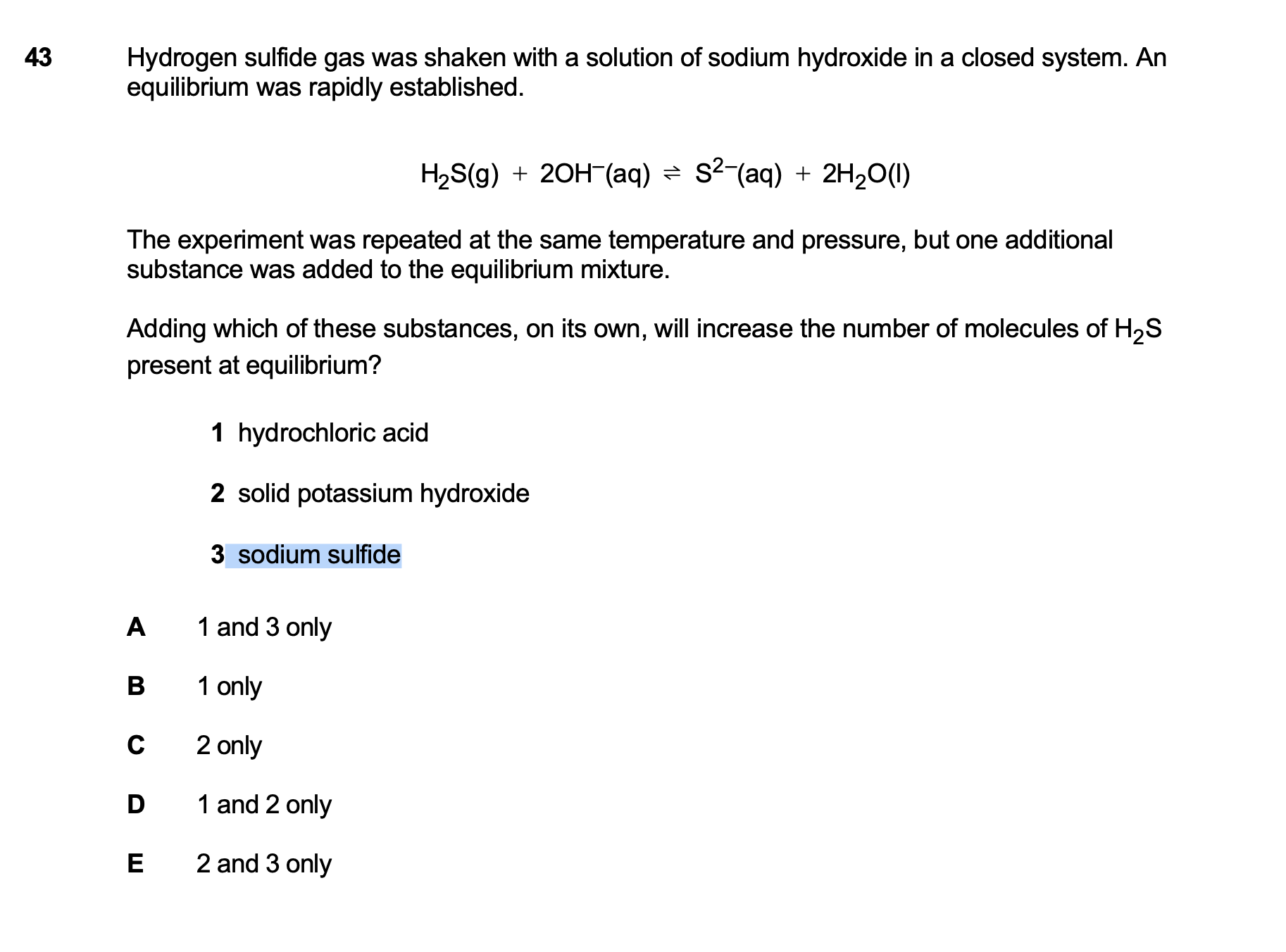
Hi! I do not fully understand, how to answer the question here, I answered it based on excluding the answers that I knew were 100 percent incorrect. Like, if I will add sodium sulfide to the solution, I will increase the concentration on the right part of equilibrium, that means it will shift to the left. Likewise, if I add hydrochloric acid it will dissociate into H+ and Cl-, hydrogen will most probably connect to the S2- and will create the H2S. How to properly solve this question? Thank you
hello!
could you give the answer to this question? thanks! also which year is this one
Hey! The answer is A and it is IMAT 2022
Guys! You forgot that HCl is an acid, and OH- is basic. We also have that as part of our equilibrium!
could you explain this a little bit more? is the answer wrong or did i give missing information? i couldn’t get it, thanks
Adding an acid will cause it to shift to the left, which contains a base (OH-) to make sure we maintain the equilibrium
Then, is Nihan’s reasoning correct, since acids (HCl, Na2SO4) will demolish 2 hydroxides and cause an increase in moles of H2S?
yes! the H+ will react with OH- causing a decrease in OH- causing a shift to the left side. just forgot to mention that it is an acid base reaction. instead of saying the whole reaction with OH-, I could’ve used acid base reaction in shorter terms
na2so4? does this result from the reactions or did you mean statement 3 which is Na2S?
no worries thanks for clarifying
