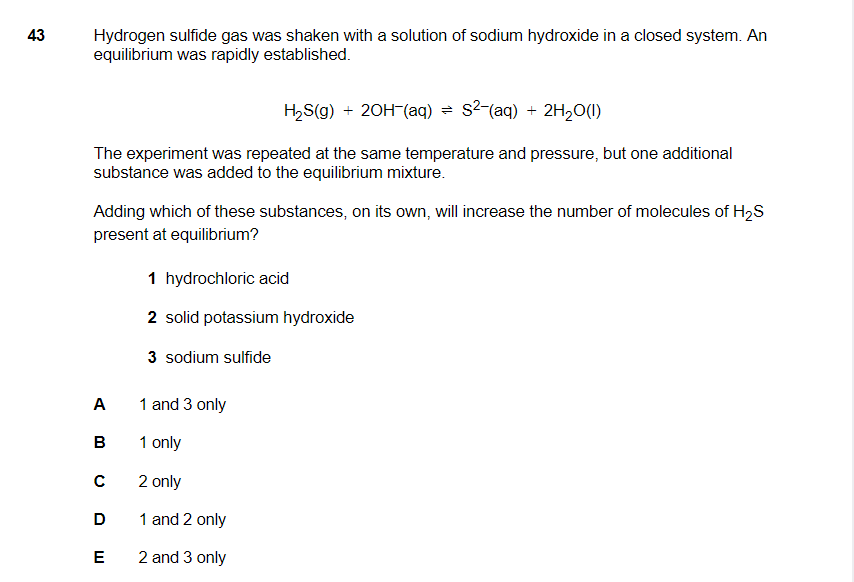Hello!
for the 1st one: if you add HCl into the solution, the solution will become acidis as it dissolves and gives H+. In this case, in order to balance it the equation will shift to the left hence increasing the h2s concentration.
3rd one: when sodium sulfide dissolves it will give of S ions. increasing of S in the right. shift to the left
(correct me if I am wrong but this is what I remember)
Thank you, Nihan! Now I understand
1 Like