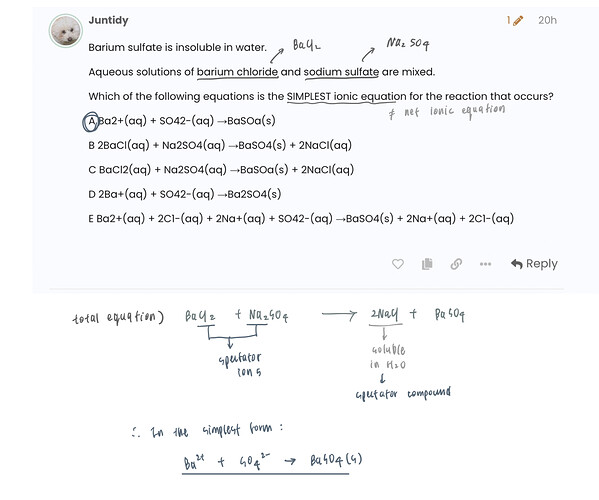
Barium sulfate is insoluble in water.
Aqueous solutions of barium chloride and sodium sulfate are mixed.
Which of the following equations is the SIMPLEST ionic equation for the reaction that occurs?
A Ba2+(aq) + SO42-(aq) →BaSOa(s)
B 2BaCl(aq) + Na2SO4(aq) →BaSO4(s) + 2NaCl(aq)
C BaCl2(aq) + Na2SO4(aq) →BaSOa(s) + 2NaCl(aq)
D 2Ba+(aq) + SO42-(aq) →Ba2SO4(s)
E Ba2+(aq) + 2C1-(aq) + 2Na+(aq) + SO42-(aq) →BaSO4(s) + 2Na+(aq) + 2C1-(aq)