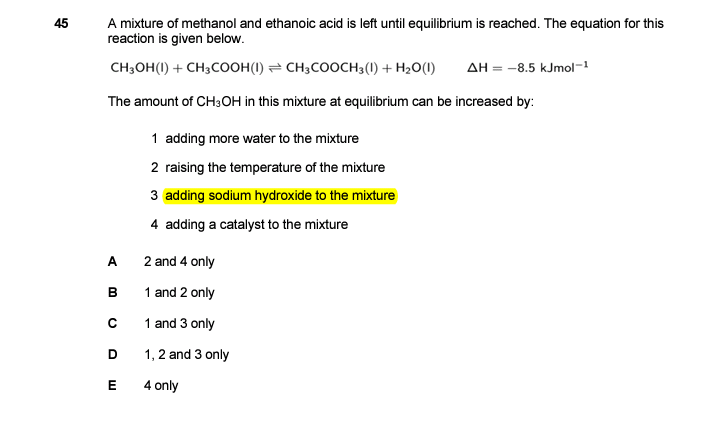
Hi! sodium hydroxide is a base so making the solution more basic would shift the equilibrium to the left to decrease the pH which would produce more of methanol
hi! if we put NaOH it will ionize in water and give OH molecules. if the system’s OH molecules get high, according to Lchatelier’s principle, the system will shift to the side where fewer OH molecules are around. this means to the side of reactants because OH is in the compounds. so they won’t dissolve as much and the reaction shifts to the left.
so 3 will be included because it will cause CH3OH to increase
am I correct? please let me know!
I think this could also be understood by another way. As we add NaOH (base) to the mixture it would react with the acetic acid (CH3COOH) present in it to form sodium acetate (CH3COONa) and (H2O).
NaOH+CH3COOH —>CH3COONa+H2O
This results in decrease in the concertation of the acetic acid (CH3COOH) as it is being consumed from the reaction mixture and increase in concentration of water. Both of these conditions will push the reaction in backwards direction, formation of CH3OH.