In the Basics part 1 section of the course, lesson 6, minute 1:20 and 14:53, Ari says that ionic compounds don’t have intermolecular forces between them because one atom transfers electrons to the other completely and no sharing occurs. But then, in the Basics part 2, lesson 11, He goes on to talk about the ionic bond which is an intermolecular force that takes place in ionic compounds like NaCl. I am confused. Can anybody please clear this up for me.
That’s odd, maybe he meant to say in basics part 1 : “ionic compounds don’t have INTRAmolecular forces between them” ?
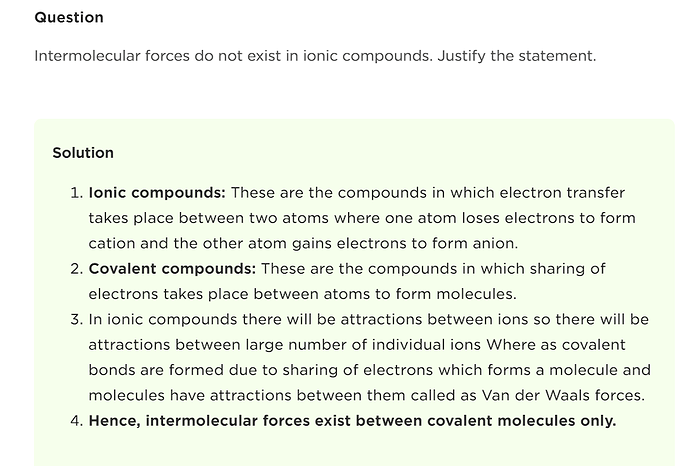
I thought so too, BUT, it’s actually explicitly stated in the practice questions section, here:
the icing on top is that I found this question on the internet with varying answers:
EDIT: I know quora isn’t the most credible place to get your information from but that’s all I could find.
Honestly the more I look into it the more confusing it gets. I’ll skip it for now and move on to other things.