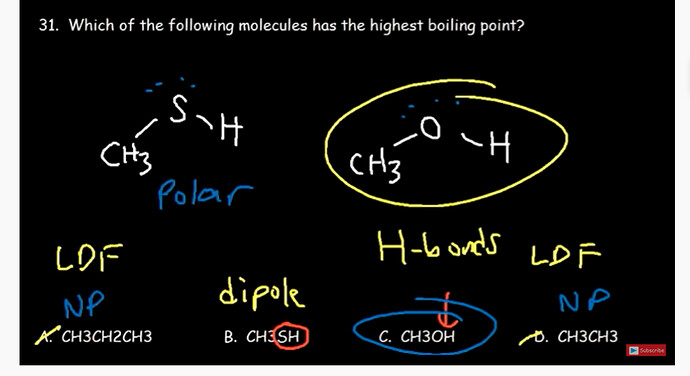
Hydrogen bonds are stronger than dipole and dipole are stronger than london dispersion force
but CH3SH also has H in this like CH3OH but it is considered that CH3SH has dipole force
Why B is polar and C not?
I believe there’s a mistake. methanol (C) is also polar as you can see that oxygen has more electron density due to its two lone pairs of electrons. This causes a net dipole pointing towards the Oxygen atom, making CH3OH polar. For B, carbon and sulfur have similar electronegative so the substantial dipole moment is due to the polar S-H bond. Hope this makes sense! Remember if the charge doesn’t cancel out in the directions of the bonds of the shape, it is polar.
4 Likes
then what’s the right answer?
C due to the H bond in O-H, remember H bonds don’t form with sulfur. And for the other two molecules they’re bonded to carbon, non polar so they’re weaker bonds.
1 Like