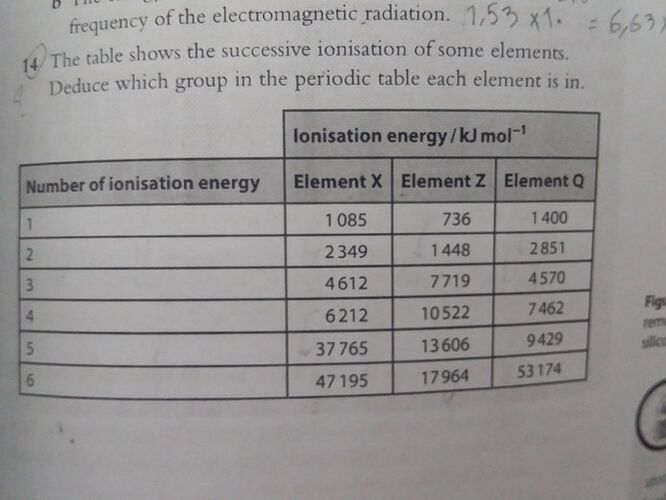
Hey! What is the solution ? I just could understand the group of element X and I didn’t have any ideas for the rest of them…
@AriHoresh
For almost all elements the group no is equal to the number of electrons in the outer most shell for example group 1 elements have 1 electron in their outer most shell and so on
And when a shell vanishes or ends due to the removal of all electrons from the shell then tge ionization number increases significantly.
In case of element X there is a sudden increase in ionization energy as we move from 4 th to 5th I. E it means that it it have 4 electrons in its outer most shell. So
X belongs to 4 th group
Z belongs to 2nd group
Q belongs to 5th group
And i have organized the group numbers according to main groups like
H group 1
Be group 2
B group 3
C group 4 and so on
1 Like
Thank you so much ![]() ⚘:star2:
⚘:star2:
1 Like