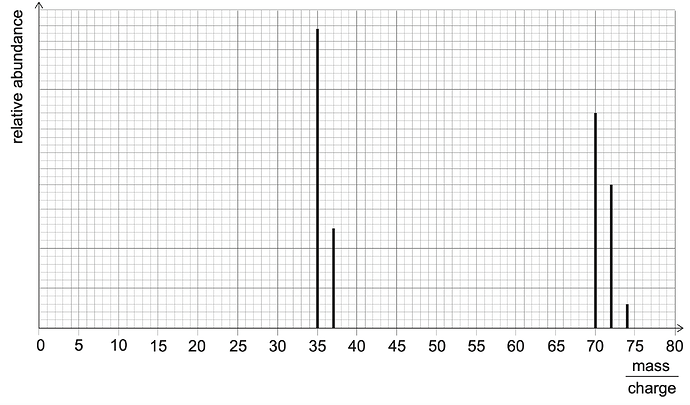
The masses of atoms and molecules can be determined using a mass spectrometer. The masses can be shown as a series of peaks.
Element X exists as a diatomic molecule. The mass spectrum will show the mass of ions from individual atoms of X and from X2 molecules.
The mass spectrum for element X is shown below:
How many different isotopes of X can be determined from this spectrum?
A 1
B 2
C 3
D 4
E 5
The answer is B 2, but I don’t really get it. Any help would be appreciated!