Hi
Can you explain why only NaCl solved in water will produce natural solution but not the other alternatives? (Source: IB)
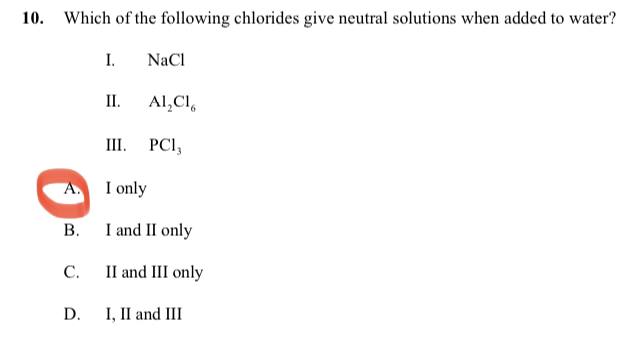
i want to know the explanation of this too, is it in any way related to the charge of the positive ions of the given options?
Hi!
To rule out II and III, starting with II first we can say:
AlCl_3 or Al_2Cl6 is covalent but in water, it becomes ionic due to large hydration energy of Al^{3+}. When dissolved in water, it becomes-
{AlCl_3 + 6H_2O → [Al(H_2O_6)]^{3+} + 3Cl^-}
That extra charge pulls electrons from the water molecules with strength towards the aluminium. That makes the hydrogens more positive and easier to remove from the ion. So, this ion is much more acidic.
The equilibria also lie further to the right, and so the solution formed is more acidic - there are more hydroxonium ions in it.
And because of the heat produced in the reaction and the concentration of the solution formed, hydrogen ions and chloride ions in the mixture combine together as hydrogen chloride molecules and are given off as a gas. With a large excess of water, the temperature never gets high enough for that to happen - the ions just stay in solution, making it an acidic solution- not neutral.
Now for III, Phosphorus Trichloride, it will react violently with water to form Phosphorus acid and Hydrochloric Acid (in gas form):
{PCl_3 + 3H_2O ⟶ H_3PO_3 + HCl}
Definitely not neutral either,
So the answer is A.
I hope this helps, and please correct me if I am wrong ![]()