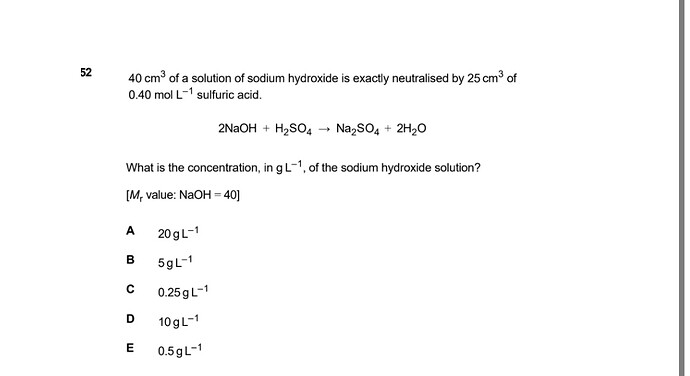
Can someone explain step by step please? Thank you
hey
we need to find the moles value for both H2SO4 and NAOH
0.4mole /1000cm3 = X mole /25 cm3
X=0.01 mole H2SO4
we have 2 moles of NAOH for one mole of H2SO4 based on the question
and each moles of NAOH is 40 g
0.01 * 2 * 40 g =0.8 g we have 0.8 g in 40 cm3 , how much in one litre = 1000cm3?
0.8 g / 40 cm3 = X g /1000cm3
X =0.8 *1000 /40 = 20 g/L
hope to be helpful !
Hi!
i’m a bit confused from that last step, how do you go from mol/L to g/L?
what I meant was that when we have 0.01 mole H2SO4 , we can calculate the moles of NAOH ,which is twice of the moles of H2SO4 =0.02 mole NAOH
THEN we have 40 g per 1 mole of NAOH SO
0.02*40 = 0.8 g NAOH , BUT this amount is in 40 cm3 as the question says that 40cm3 is the exact amount that neutralised the the initial 25 cm3 of sulfuric acid. SO at the end the calculated number of grams is in 40 cm3 , which is measured based on the primary provided data
ultimately, I mentioned that(( 0.8 g /40cm3 =X g /1000 cm3 ))to calculate the number of grams in 1 litre instead of 40cm3 .
hope it was explicit