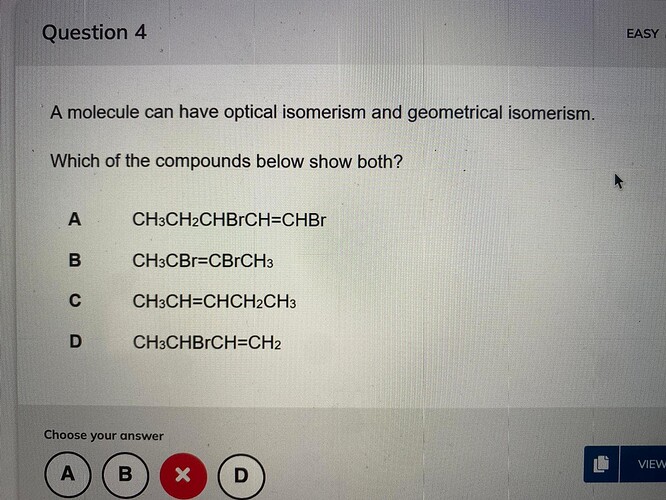
Answer is credited to @Asafmen
It depends on the cell. The more “classic” approach is of the voltaic cell in which the anode is negative and the cathode is positive (same prefixes as the ions). Electrolytic cells are the opposite.
You need the compound to be both in and out to be an isomer when you have a double bond. That means, bound with the main polymer and also integrated within it.
As a rule of thumb, it can be a geometrical isomer if they have the same compound, but the optical one has to have chirality, meaning the central carbon has to be bounded to 4 different groups to achieve that.
Something that is chiral, since everything but B can be a geometrical one.
It is a slightly problematic question since the best way to solve is to draw the molecules.
But A seems like the most convenient chiral central carbon.