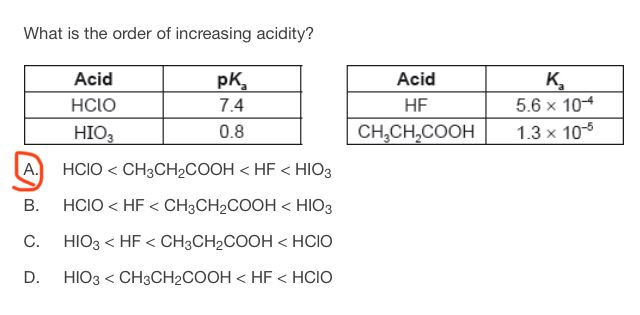
This is how I thought when I solved this question: the first table has pKa values. So the lower the pKa is, the more stroger is the acid. Thus:
HIO3> HClO
The second table has Ka values. The higher the Ka value is, the more stronger is the acid. Thus: HF> CH3CH2COOH.
When I looked at the different choices, I chose A because it was the only choice which said that HF is stronger than CH3CH2COOH. Have I missed anything in my reasoning?
D. could also be true if first and fourth acids changed places*
Answer credited to @Asafmen
The smaller the PKA, the stronger the acid.
This is because of the dissociation rate, with a maximum of 1, and a minimum of 0.
PKA on the other hand is the negative logarithm of KA.
A lower PKA indicated a stronger acid because for a small KA (weak acid) the negative logarithm will be greater.
According to that logic, HICO is the weakest acid, and {HIO_3} is the strongest.
That alone eliminated C&D. The KA of HF is bigger, meaning it is a stronger acid.
Making the correct answer A.
1 Like
Acid strength & Pka values are inversely related - lower the Pka, stronger the acid
