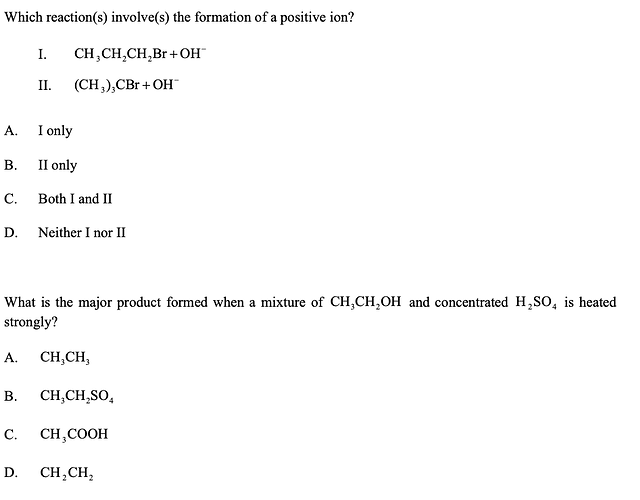
first one is B
doesn’t both reactions give rise to Br-? in this case how is the second one has a positive ion?
second one is D
First one, this might be about carbocations and their stability? Might be a reach though, if someone could help - but was thinking, mid reaction, the 2nd might actually form a partially stable tertiary carbocation whereas the first reaction would just have the substitution directly - thats the only distinction I can make to get to the answer.
i checked and it was about nucleophilic reactions but the answer still did not convince me because both had Br- as products anyways
But surely the OH- ions aren’t added by themselves? They will be added as part of eg. KOH, so surely the Br- will react with the K+ and KBr will be formed anyway? Im just trying to nitpick tbh and say that because it says ‘involves the formation of a positive ion’ and thats all I could think of.