this is my first time seeing a question like this one, which topic is this and how is this solved?
thanks in advance
I don’t know how to solve this.
But this question has been taken from spectroscopy topic (Chapter 18: carbonyl compounds of A-level book)
Hi!
(i also haven’t studied this in a few years so please fact check me)
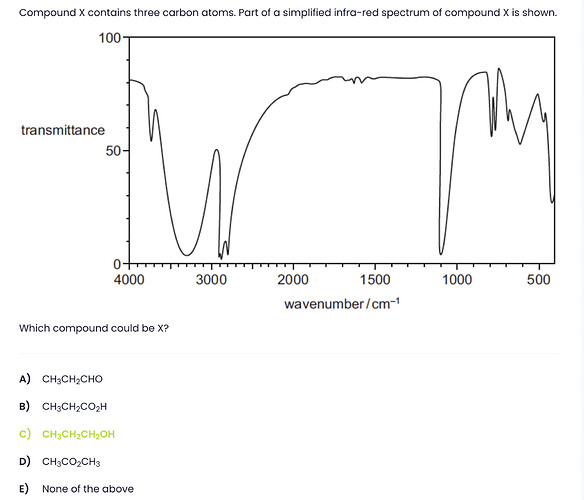
this is infrared spectroscopy, each dip on the analysis corresponds to a specific bond (for example O-H in an alcohol is specifically 3200-3400)
I’m thinking they cannot possibly expect us to know the range of every bond possible, even in highschool we we’rent expected to.
Luckily this question can be answered with general knowledge about IR spectroscopy.
- ignore the values below 1500, this is the molecules “footprint”
- from 1500-1800 if we have an “icepick” it means we have a double bond in the molecule (we have NONE here)
- an “icepick” around 2900 means we have C that has sp³ so simple bonds (we have some here)
- from 3000-4000 if we have an “icepick” it means there’s a simple bond X-H (we have one here)
- from 3000-4000 if we have a “valley” (a larger dip than an “icepick”) it means there’s a X-O bond (we have one here)
now: draw out the structure of every molecule
we know there’s an O in the molecule, and they all have one
we know there’s NO DOUBLE BOND so the answer cannot be A) has an aldehyde, or B) has a carboxylic acid, or D) has an ester
We are left with C) has an alcohol
I hope my explanation is clear enough!