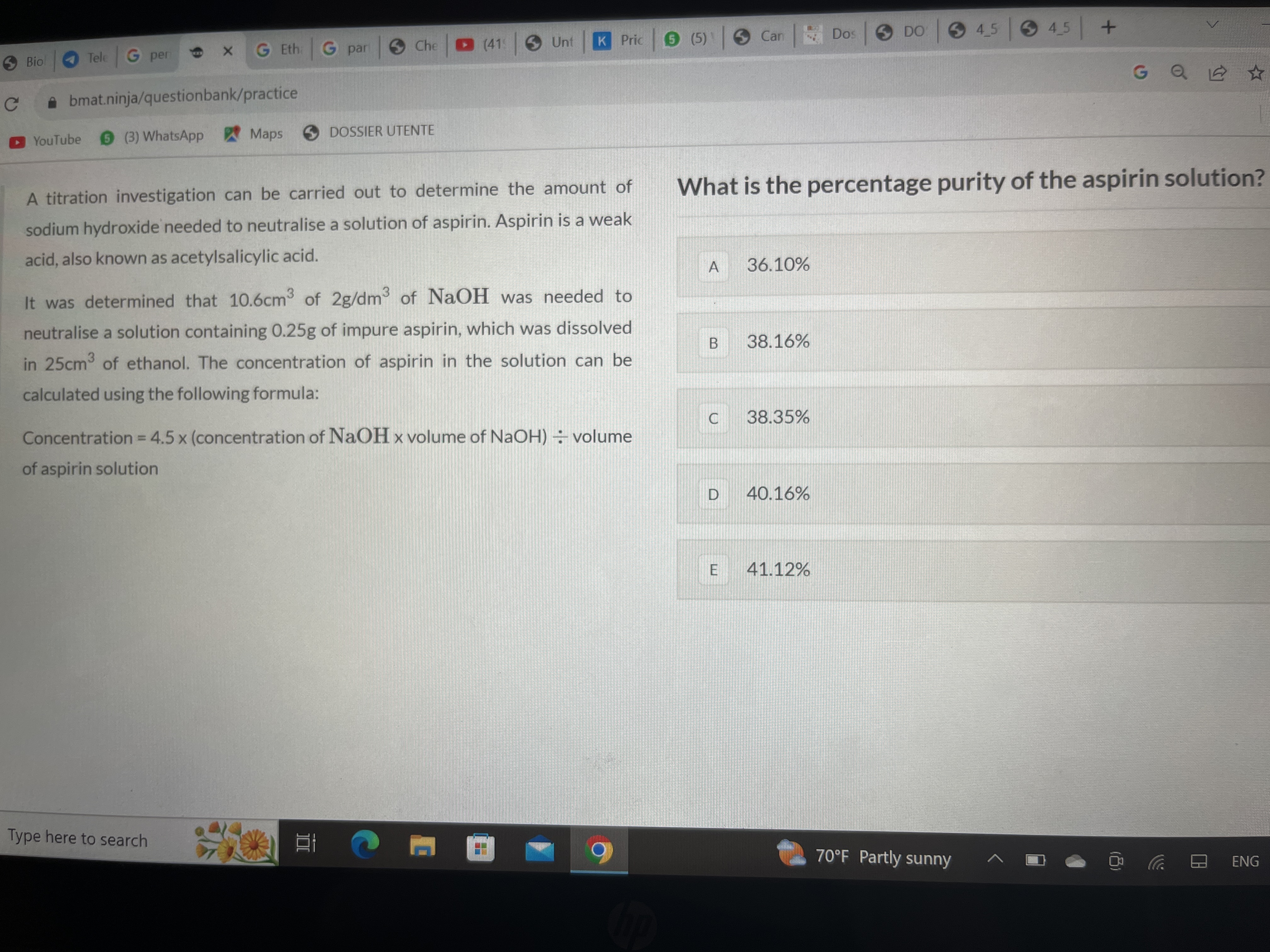
Hello anyone can help me solving this question?
Hi, if you first use the formula given in the question:
Concentration of aspirin = 4.5 x 2 g/dm3 x 0.0106 dm3(converted from 10.6cm3) ÷ 0.025 dm3(converted from 25cm3)
= 3.816 g/dm3
Using this aspirin concentration to find the mass of pure aspirin dissolved in 25cm3 of ethanol:
0.025 dm3 x 3.816 g/dm3 = 0.0954g
Now that we know the mass of pure aspirin and impure aspirin, we can find the percentage purity of the aspirin solution:
0.0954g / 0.25g x 100 = 38.16%
3 Likes