hello everyone, i have a question in chemistry
So , when we have to find if a molecule is polar or non polar
Do you know an easy way to do it?
I know we can draw lewis structure and see the lone and bond pairs but this doesnt take time?
i saw a helpfull video on this topic (i will post the link) but im not very sure if i completely understood it
can you explain what do you mean by electron density?
i have in my mind these,i dont know if i have understood it correctly
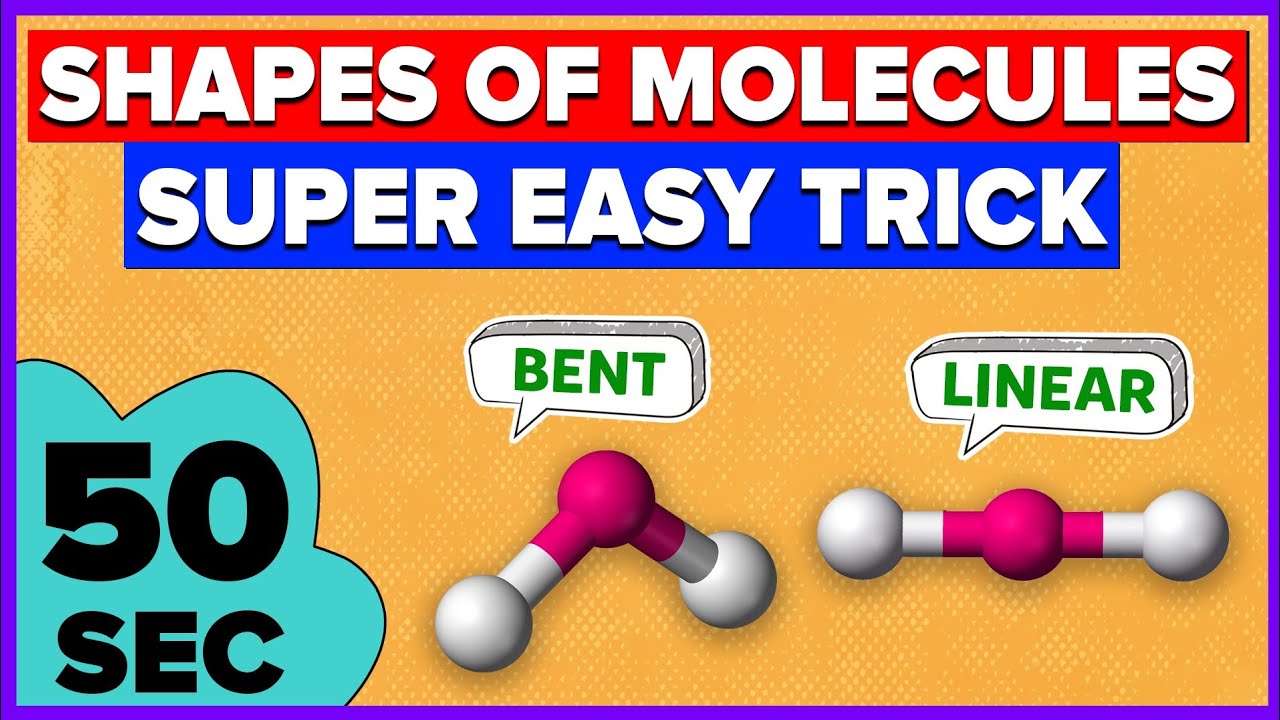
linear=180 degrees=non polar
v shape/bent=104,5=polar
trigonal pyramidal=107=polar
trigonal planar=120=non polar
tetrahedral=109.5=can be non polar or polar
and that the most of the times but not always C,Be,Al,Si form non polar and that P,S,Se form polar compounds
can you correct me if im wrong?
thank you so so much for your help have a good day!!!
1 Like