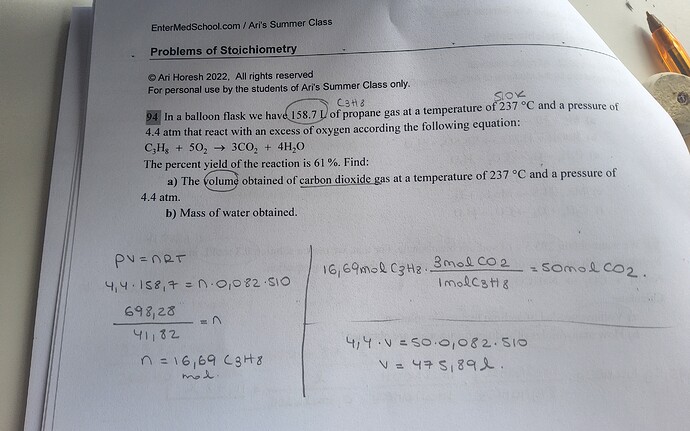
Hii!! Can someone help me to find the moles of CO2 to find the volume?
Thank you in andvance ![]()
Hey!
You were on the right track, but dont forget pressure must be in kPa in ideal gas law formula. So 4.4 atm = 4.4 * 10^5 Pa = 4.4 * 10^2 kPa.
Also, the R constant (ideal gas constant) is 8.31. So try to use it to find moles of propane and then multiplied by 3 will give you the moles of CO2. When you substitute all the numbers to find the VOLUME of CO2, multiply the number by 0.61 as the percentage yield is only 61%.
Hope that helps:)
4 Likes